
This process is emission-free and offers the potential for reducing greenhouse gas emissions. Electron Configuration: 1s1: Possible Oxidation States +1,-1: Electron Affinity kJ/mol 72.8: Electronegativity Pauling scale 2. It can be used as a fuel for fuel cell vehicles, where it combines with oxygen from the air to produce electricity, with water being the only byproduct. Since the number of electrons is responsible for the chemical behavior of atoms, the. Hydrogen also holds promise as a clean and sustainable energy source. Period A horizontal row in the periodic table. Members of a group typically have similar properties and electron configurations in their outer shell. It is used as a fuel for rocket propulsion, in the production of ammonia for fertilizers, in the petroleum refining process, and in the manufacturing of electronic components. H Hydrogen 1 1.008 Glossary Group A vertical column in the periodic table.
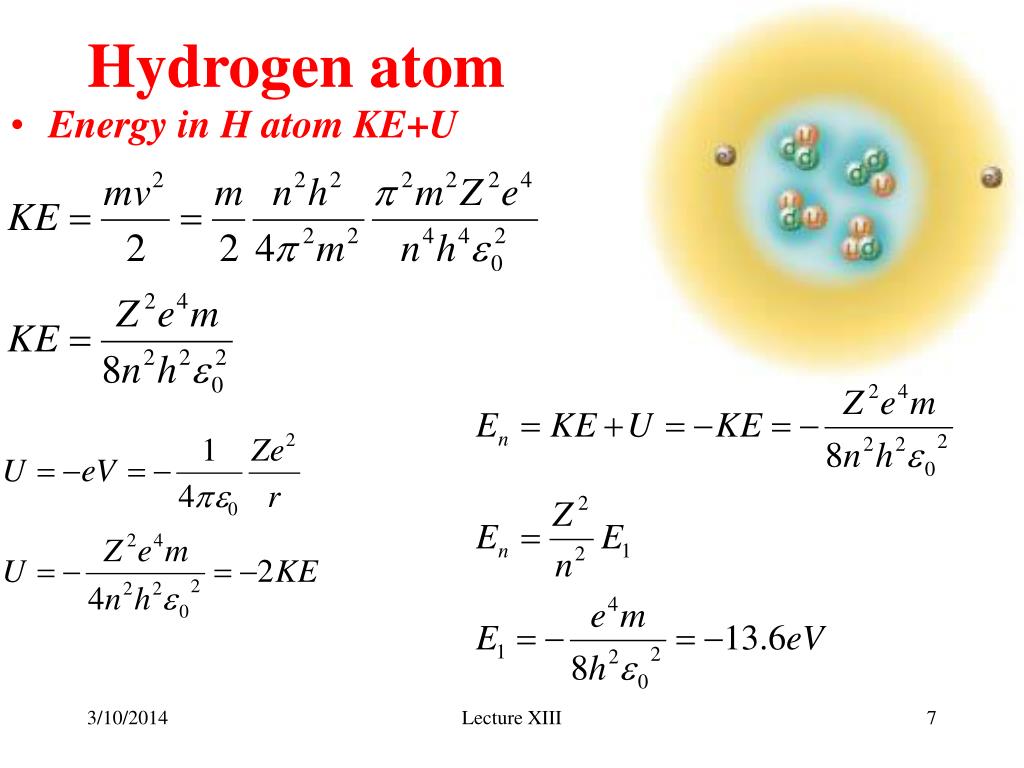
Hydrogen has numerous applications and is used in various industries. Hydrogen is found in a variety of compounds, such as water (H2O) and hydrocarbons. It is the simplest and most basic element, making up about 75% of the elemental mass of the universe. Hydrogen has a single proton and a single electron. Overview of Electron Configuration Of Hydrogen Atom The arrangement of electrons in its shells and subshells according to the energy that they have, is attributed to the work of various famous scientists and chemists of this field. Hydrogen is a colorless, odorless, and highly flammable gas in its pure form. A hydrogen atom consists of one electron which is represented as 1s1. It is the lightest and most abundant element in the universe. Hydrogen is the first element on the periodic table with the chemical symbol H. Some interesting facts of Hydrogen are given below – With the atomic number one, there is one proton in the nucleus and one electron orbiting the nucleus of a normal (electrically neutral) hydrogen atom. Hydrogen has density ‘0.09’ and it is found ‘0.14’% on earth. Question : write some information about Hydrogen ?Īnswer : Hydrogen has melting point = -259 The third major category of elements arises when the distinguishing electron occupies an f subshell. These are In the Bohr model, electrons exist within principal shells. Question : write the electron configuration of Hydrogen element ?Īnswer : Hydrogen electronic configuration is ”1s1”. Three concentric circles around the nucleus of a hydrogen atom represent principal shells. Answer : as we know Hydrogen element is denoted by ‘H’ symbol and Hydrogen has ‘1.008’ atomic mass and ‘1’ atomic number.


 0 kommentar(er)
0 kommentar(er)
